A COMPARATIVE TRIAL OF THE POLLEN SUBS
Part 6: DO WE NEED TO REVISE DE GROOT?
Beekeeper-Funded Research
First Published in ABJ December 2021
Randy Oliver
ScientificBeekeeping.com
Bee nutritionists have long cited the essential amino acid ratios suggested by de Groot in 1953. But he himself pointed out the shortcomings of his methods and qualified his recommendations. I feel that after nearly seventy years, it may be time to catch up with the other livestock industries, and update our knowledge on what constitutes an optimal supplemental diet for honey bee colony growth.
De Groot understood that what really counted for bee diets intended for colony growth would be the requirements for the nurse bees producing the jelly necessary to feed the larvae, queen, and older workers. But that’s not what he investigated. This has bugged me for years — that few researchers other than Elton Herbert have followed up on measuring the growth of colonies (as opposed to a few caged workers) fed artificial diets, which would be the best and easiest way to determine whether de Groot’s essential amino acid (EAA) ratios for adult (non-nurse) bee development would be the same as for overall colony development, which is what we beekeepers would want in our pollen subs. In this last article of this series, I’m going to stick my neck out and make a stab at revising de Groot’s ratios and suggest the sort of applied research that our industry deserves.
Allow me to begin by taking a look how the amino acids in a diet are actually used by an organism.
HOW PROTEIN AND AMINO ACIDS ARE USED
The body tissues of adult bees are largely composed of protein — proteins constitute about 50% of a bee’s dry weight [[1]]. The various types of body and haemolymph proteins are all created from the metabolic pool of amino acids derived from their diet, as illustrated by Figure 1 (published during the years in which de Groot was performing his research).
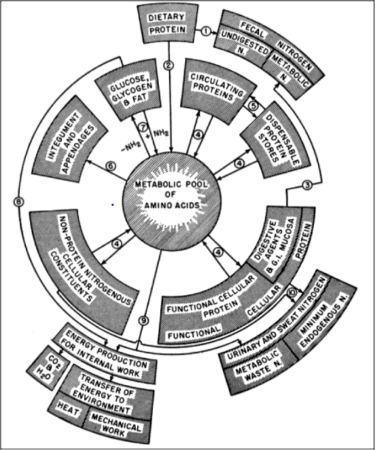
Fig. 1 Dr. Harold Mitchell was a pioneering animal nutritionist at the University of Illinois. In 1950 he published this flow chart of how dietary proteins were used by the body. Image from [[2]].
The protein in a diet is first digested into its constituent amino acids, from which the body then synthesizes new body components by the process of anabolism (the opposite is catabolism — the “destructive metabolism” of body tissue). That process is limited largely by the amount of essential amino acids (EAAs) in the diet [[3]], as well as their relative proportions to each other. The EAAs are used not only for building (or replacing) muscle mass and internal organs, but also to create haemolymph and dispensable storage proteins. Some protein is interspersed in chitin to form the bees’ cuticle and wings. And some may be burned as fuel for energy if enough carbohydrates (sugars) are not available in the diet.
One way to measure bee growth is by drying and weighing individual bees. But such weight gain includes components largely built from carbohydrates, such as blood sugars, low-proteinaceous chitin, and lipids. With regard to artificial diets, what we’re more concerned with is the utilization of the nitrogen in the diet, which comes from the protein.
Practical application: So perhaps what we really should focus upon is their increase in nitrogen content.This consideration did not escape de Groot, so he measured both dry weight gain as well as nitrogen increase (Figure 2).
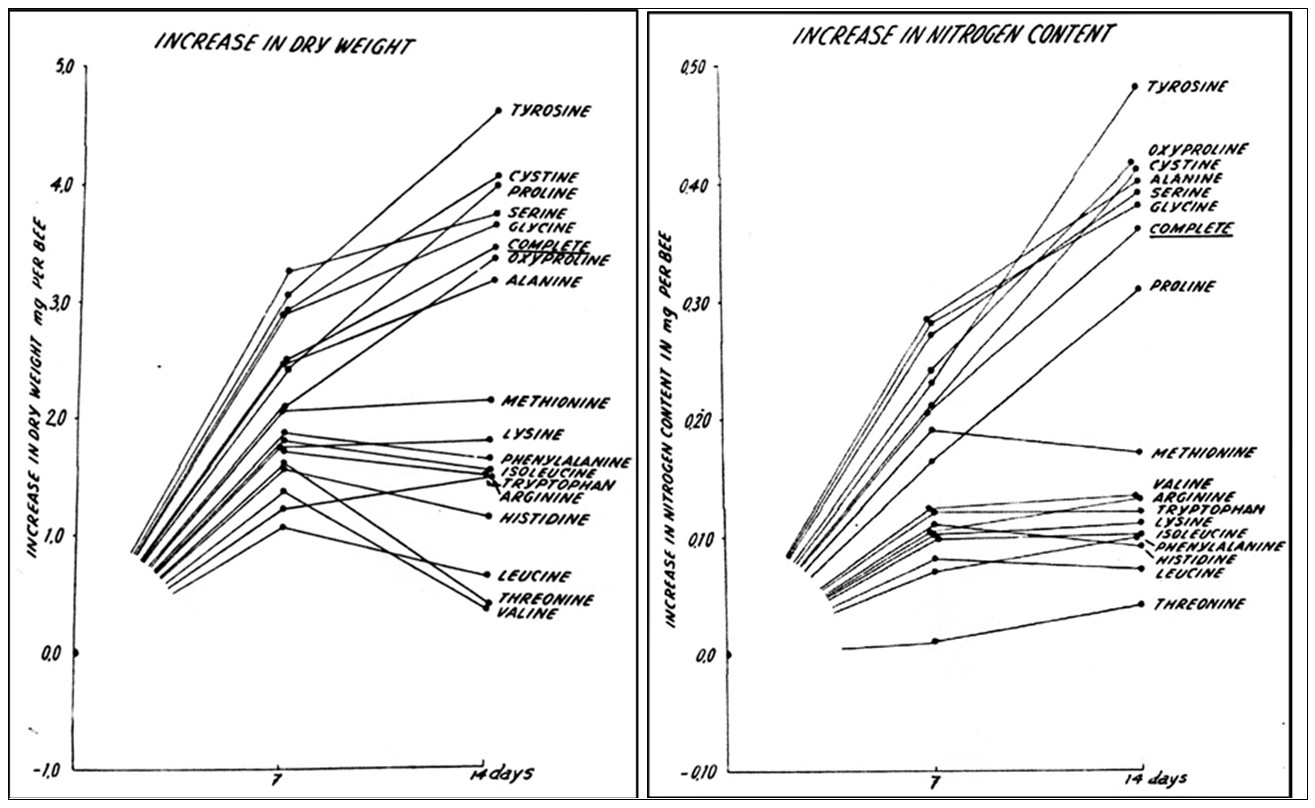
Fig 2. Compare the effects of EAA deficiencies upon weight gain (above) versus gain in nitrogen (below), which indicates the actual incorporation of those EAAs into body mass. Note severe weight loss in adult bees if leucine, threonine, valine are deficient. But the graph at the right indicates that the weight loss was due to the critical requirement of those EAAs for the process of building non-proteinaceous body tissues, rather than being incorporated into those tissues.
Practical application: We can’t just look at protein analyses of body tissues estimated from nitrogen content; we must also keep in mind that some EAAs are essential for the building of non-nitrogenous body components. I’ll return to this concept when I discuss “carcass analysis.”
NUTRIENT REQUIREMENTS CHANGE WITH STAGE OF DEVELOPMENT
At each stage of development, a worker honey bee is anabolizing different body components, perhaps consuming different food, and may require different proportions of EAAs:
- Larval development — Major weight gain consisting of a small amount of integument, muscle, digestive tract, and tracheal system, but mostly from large amounts of storage protein in fat bodies [[4]]. Protein source — nurse-fed jelly.
- Pupal-to-adult development — Development of the chitinous exoskeleton (including wings, legs, and eyes), adult organs, and body musculature. Protein source — the fat bodies of the pupa, and the repurposing of pupal tissue. This means that the pupa is its own protein source, so that analysis of the amino acid ratio of its body may be informative.
- Post-emergence development — Major development of the hypopharyngeal glands and fat bodies, Partial development of the wing muscles and the sting. Protein source — pollen and some jelly.
- Nurse bee development — The nurses need to produce copious quantities of protein-rich jelly for feeding the larvae and other members of the colony. Protein source — pollen.
- Mid-age worker development — Development of the wax glands and wing muscles. Protein source —pollen, jelly, and autophagy of the fat bodies and hypopharyngeal glands as they lose weight in preparation for foraging [[5]].
- Forager development — Final growth and maintenance of the wing muscles and body tissues. Protein source — jelly begged from nurses.
- “Winter bee” development — The diutinus state taken by workers that emerge during pollen dearths might be considered as “arrested development” of nursing physiology. Protein source — pollen.
- “Winter bee” maintenance — Very little protein is required. Protein source — a small amount from honey, stored beebread, autophagy (“self-consumption” of storage proteins in the fat bodies) [[6]].
Practical application: Similar to the different diets sold for the optimal development of broiler chickens at each stage of growth, we may find that there is no one-size-fits-all diet for honey bees.
WINTER FEEDING
AUTUMN FEEDING FOR STORAGE PROTEINS
Before I return to diet for colony development, let’s first consider diets in preparation for, or during winter. Beekeepers feed pollen subs not only to provide the necessary nutrients for the last rounds of brood that will become long-lived “winter bees,” but also to allow those emerged workers to fully develop their storage proteins in order to survive the winter. Would the development of storage proteins require a different EAA ratio in the diet?
As pointed out by de Groot:
Many kinds of insects can maintain life for considerable periods in the absence of nitrogenous food.
They do this by digesting their body protein reserves (catabolism). “Winter bees” exhibiting diutinus physiology (similar to nurses, but not producing jelly), as mentioned earlier, break down their protein reserves in order to obtain the amino acids (and under conditions of starvation, energy) necessary for “body maintenance.”
Practical application: De Groot studied the EAA requirements of young workers not being exposed to brood pheromone, suggesting that they might have gone into diutinus physiology. Thus, his recommended EAA ratios may well apply to diets fed to the last round of emerging workers after broodrearing ceases in autumn.
Many beekeepers feed pollen subs in autumn in order to prepare the “winter bees” for longevity and late-winter brood rearing. Unlike as with forager-collected natural pollen or dry-fed pollen sub, diets fed in patty form inside the hive are not stored in the combs [[7], [8]], so the benefit of autumn feeding of pollen patties may mostly be to promote the synthesis of those storage proteins. That said, those storage proteins allow for only a very limited amount of brood rearing. Haydak’s [[9]] findings suggest that it requires the storage proteins of around 30 worker bees to rear a single replacement worker to adulthood (at which point that worker would still need to consume additional protein in order to complete its development).
Most beekeepers have heard of the important storage protein vitellogenin. Of interest is that Otis [[10]] found that winter bees stored more of a hexamerin protein (presumably arylphorin) than vitellogenin. They also confirmed that a “winter bee” stored only a fraction of the protein necessary to feed even a single larva.
So which EAAs are important for the formation of these hexamerin storage proteins? Those rich in the aromatic amino acids (phenylalanine, tyrosine, and tryptophan) are called arylphorins, whereas another group of hexamerins are known as methionine-rich storage proteins. Frustratingly, I was unable to find information on the EAAs in vitellogenin. The extremely high proportion of tyrosine in insect storage proteins [[11]] suggests that we might want to pay attention to this “conditionally essential” amino acid in diets intended for colony development or “winter bees.”
Practical application: The findings of Haydak, Jeffree, and Otis point out that the ability of colonies to rear brood during winter is poorly understood. We clearly need more research on this subject.
WINTER FEEDING FOR BODY MAINTENANCE
De Groot found that based upon his preliminary research:
From the above results it may be concluded, that in contrast with findings of other authors the longevity of old bees is increased by supplementing the carbohydrate diet with various protein containing proteins or pure protein in a suitable concentration. Obviously in the bee colony the need for protein is not restricted to the brood rearing period but proteins are required for maintenance of adult bees as well. This finding is in striking contrast with the widespread opinion, that during the winter period the bee requires only carbohydrates. It is common use in beekeeping practice to substitute the honey for stores of pure sugar for overwintering bee colonies, which is not considered to be disadvantageous from the nutritional point of view. However, honeys contain small amounts of protein varying in the region where we found the protein containing material to exert its longevity promoting action on old bees. … Therefore it is obvious to suggest that sucrose stores enriched with a suitable amount of protein food with a high biological value may be more favorable for overwintering bee colonies than [the] stores of pure carbohydrate generally used at present in apiculture.
Winter feeding with straight sugar is still a common practice today (Figure 3).

Fig. 3 A wintering colony being fed dry granulated sugar. This method was described by Johansson in 1977 [[12]], and later popularized on Beesource as the “mountaincamp method,” after posts by a beekeeper from Mountain Camp Farm in New York. Note that Johansson cautioned that colonies fed with dry sugar may need to forage for water to dilute the sugar. Photo credit: Phillip Cairns/ mudsongs.org
Practical application: Adding some high-quality protein to sugar fed during winter would likely help the “winter bees” to maintain their body reserves. Several manufacturers are offering protein-enriched “winter patties.” We could sure use some easily-performed field trials to see whether enhancing that protein with the certain amino acids [[13]] would be of benefit.
WHICH EAA RATIO IS BEST FOR COLONY DEVELOPMENT?
Winter feeding aside, most of us feed pollen sub to allow our colonies to grow. Jeffree [[14]] found that colonies in Scotland enter winter without enough stored beebread to allow nurse bees to feed more than a minimal amount of larvae, yet sporadically rear larvae throughout the winter. Thus late-winter feeding of pollen sub can be of great benefit for those beekeepers who require strong colonies for early pollination contracts.
Practical application: We almond pollinators have found that midwinter feeding of pollen sub under the right conditions can encourage serious brood rearing and colony buildup.
AT WHAT DEVELOPMENTAL STAGE DO BEES MOST REQUIRE PROTEIN?
So the question is, at what stage of development does a worker bee actually require the most protein in its diet? It’s during their larval stage (Figure 4).
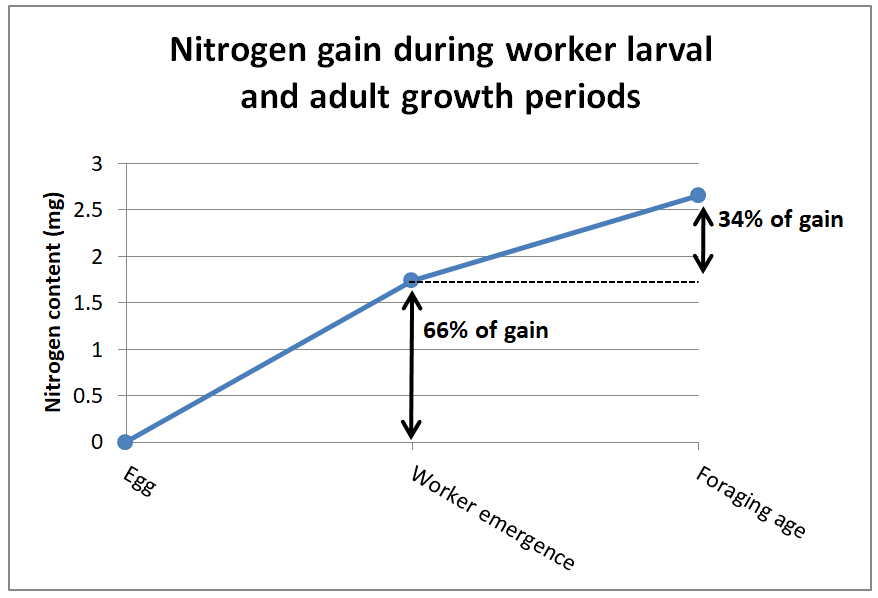
Fig. 4 Increase in nitrogen content of the bodies of worker bees during growth periods. Two-thirds of the incorporation of amino acids into a bee’s body occurs when they are consuming the jelly produced by nurse bees, as opposed to the 1/3 added from the pollen that it consumes after emergence. Data from [[15], [16]].
De Groot’s estimates represented the EAA requirements for newly-emerged non-nursing adult workers (which may have developed their ovaries due to the lack of suppressive queen pheromone [[17]]). The graph above indicates that the protein requirement of the larvae is more important as far as colony growth. But the larvae benefit from pollen sub indirectly — it is the nurse bees that actually consume the pollen sub and convert it to jelly (any pollen that the larvae might receive from the nurses provides an insignificant amount of protein overall [[18]]).
Thus de Groot’s estimated ratios may not apply to the all-important nurse bees, which are responsible for the digestion of proteins for the entire colony (and are thus the consumers of pollen sub). The nurses then convert pollen sub into the jelly to feed the queen, the brood, and the rest of the workers (jelly being the “currency of protein” within the colony).
Practical application: So for colony growth, what we really need to determine is the optimal EAA ratio in the diet of nurse bees feeding larvae.
WAYS TO DETERMINE OPTIMAL EAA REQUIREMENTS
Although researchers have now developed methods for successfully rearing worker larvae in vitro, the diet consists mainly of harvested royal jelly [[19]]. Until a completely artificial diet for larvae is developed, we need to stick to figuring out diets that allow nurse bees to produce the most jelly.
There are several possible ways for researchers to do so. Allow me to list some other benchmark indicators to which we can compare de Groot’s recommendations. I will graph them out after explaining them.
COMPARE TO ROYAL JELLY
It would be reasonable to assume that royal jelly produced by nurse bees would contain the ideal ratio of EAAs for optimal larval growth. I downloaded several analyses of the amino acids in royal jelly, and found that the EAA ratios were remarkably consistent, despite being from different countries and the consumption of different pollens. Some of the older analyses lacked data for tryptophan. Fortunately, in 2009 a new method was developed to quantify tryptophan [ [20]], so I was able to insert a value. I averaged the five most consistent analyses for graphing purposes.
Side note: An interesting study by Webster [[21]] found that nurse bees that cannibalize brood rapidly recycle the amino acids of those larvae back into royal jelly, and to a lesser extent into body tissue. The take-home is that bees don’t waste amino acids, and that open brood can serve as a protein reserve for producing jelly.
COMPARE TO KNOWN NUTRITIOUS NATURAL POLLENS
Studies from the Tucson Lab in the 1970s confirm beekeeper observations that colonies grow very well on almond pollen [[22]], so we can likely use the EAA ratio of almond pollen or other known nutritious pollens (such as mustard or sweet clover) as benchmarks.
COMPARSION TO OTHER ANIMALS AND WHAT THEY FEED THEIR OFFSPRING
Similar to royal jelly being the “perfect” food for queens and larvae, cows and chickens also produce proteins that are perfect foods for their offspring — casein and albumin, respectively. Again, both of those proteins are the result of evolutionary selection for an EAA composition that would allow for complete development of their offspring. Perhaps not surprisingly, both work very well as protein sources for honey bees [[23],[24]].
CARCASS ANALYSIS
Another possible indicator of EAA requirements for a diet are the EAAs present in the animal’s body. This is termed “carcass analysis” [[25]], and would certainly be a simple way to determine the ideal ratio. Unfortunately, EAA requirements change with an animal’s stage of growth [[26], [27]], and whether the EAAs are actually incorporated into the body, or merely involved in the process of tissue synthesis.
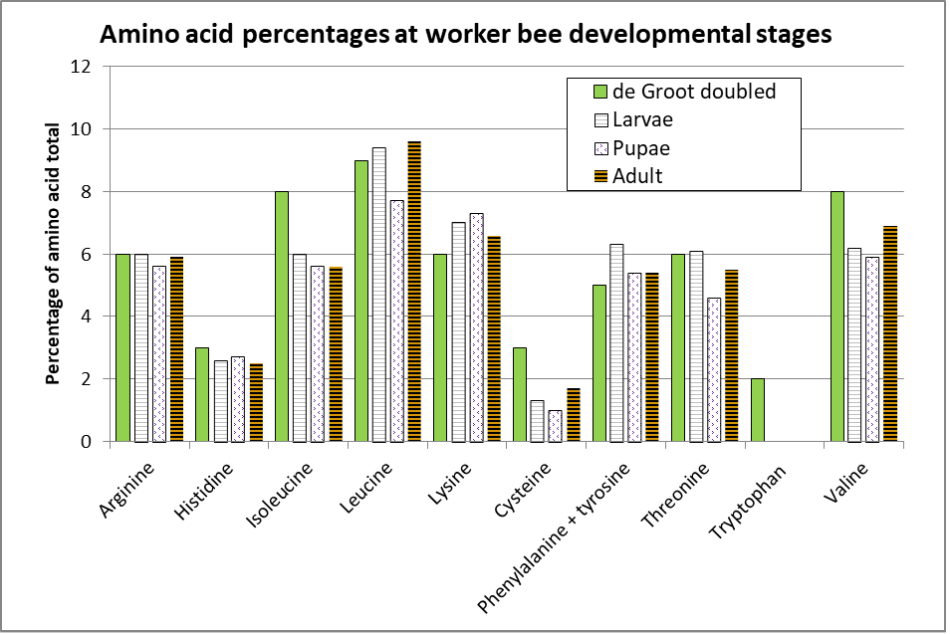
That said, Ghosh [[28]] provides amino acid analyses for worker bee larvae, pupae, and adults (Figure 5).
Fig. 5 Comparison of the EAA ratios of the three developmental stages of honey bee workers, compared to those determined by de Groot. (I doubled de Groot’s ratio, since his EAA mixture only constituted 25.6% of the protein in the diet, whereas it should likely have been ~50%.) Of interest is how the concentrations of leucine, phenylalanine, and threonine drop substantially upon pupation (after the larvae have defecated), indicating that those amino acids were necessary for tissue formation only, rather than being incorporated into the resulting body tissue.
Notes for Ghosh’s findings:
- Non-essential tyrosine and cysteine may substitute for essential phenylalanine and methionine (respectively) if they are in short supply. For some reason Ghosh did not measure methionine, whereas de Groot did not measure cysteine or tyrosine, so the comparative columns for those AAs are questionable. In many current EAA analyses, authors combine the two pairs of complimentary amino acids, as do I.
- The adult bees analyzed were in-hive “house bees” (wings removed) that had the opportunity to feed upon pollen in the combs, so their EAA changes from pupae were likely due to the development of adult tissues after feeding upon pollen.
- Tryptophan is required for tissue growth, but not so much for maintenance. Ghosh’s analysis was unable to detect tryptophan in larvae or pupae, and it barely registered in the adults — this is surprising, since de Groot determined that tryptophan should account for a full 4% of the EAAs. And remember Figure 2, which indicates that the requirements for leucine, threonine, and valine may be underestimated by carcass nitrogen analyses alone.
From the above graph, it appears that for larval growth, compared to adult weight gain, larvae require a bit more isoleucine, lysine, phenylalanine, and threonine, and a little less valine than do adult bees. Of interest is that Ghosh was not able to detect tryptophan in larvae or pupae, and only 0.03% in adults — further reason to not normalize EAA ratios to tryptophan.
Practical application: De Groot’s ratios for adult bees appear to be close to those required for larval development, but the differences indicate that the optimal EAA ratios for an ideal diet for colony growthmight need to be slightly tweaked.
I’ve since engaged in reviewing a large amount of research on the amino acid composition of natural pollens noted as being of high nutritional value to bees, analyses of royal jelly, and the amino acid requirements of other insects and animals. I’ve come to the conclusion that de Groot’s experimentally-determined ratios should be looked at compared to other findings, such as the fact that bees grow very well on the proteins casein (from milk) [[29]], albumen (from chicken eggs) [[30], [31]], royal jelly, and some pollen types. So I compared the EAA ratios of the above protein sources, as well as the EAA ratio in bee brood itself in the chart below (Figure 6).
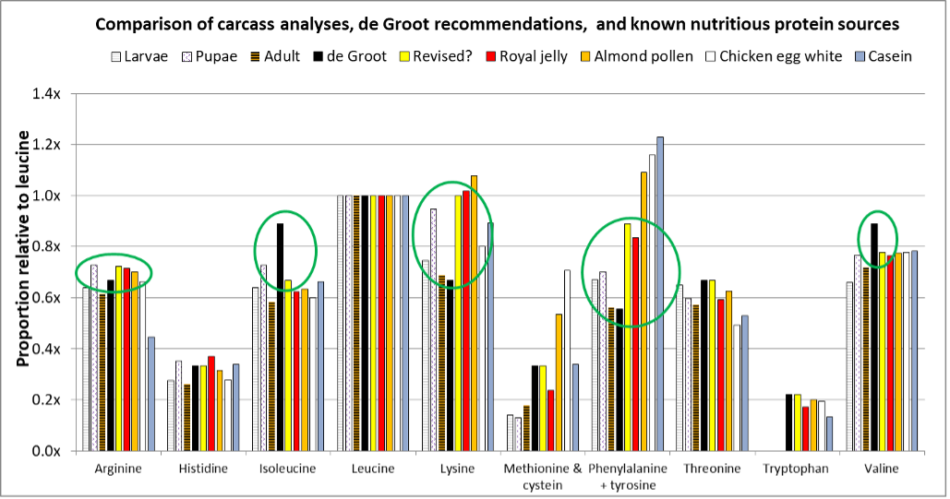
Fig. 6 De Groot’s recommendations, compared to carcass analyses of worker developmental stages, royal jelly, pollen, chicken egg and cow milk proteins (both of which have been shown to support bee growth). I’ve circled indicators of where de Groot’s EAA ratios may need to be adjusted for colony growth as opposed to emerged worker increase in nitrogen content [[32]]. I’ve also (in yellow) inserted a suggested revision of de Groot’s recommended ratios. Note that not all analyses combined the aromatic amino acids.
Practical application: The above chart suggests that de Groot was certainly not far off the mark. However, for optimal egg-to-adult worker growth, his suggested ratios may be a bit high for isoleucine and valine, and low for lysine and phenylalanine. We clearly need further experimental results to confirm. But allow me to be bold enough to suggest a revision (Table 1):
| Table 1. Suggested revisions (in red) to de Groot’s recommendations for the proportional ratios of the EAAs for diets intended for colony growth. | |||
| de Groot | Revised? | Revised normalized to leucine | |
| Arginine | 3 | 3.25 | 0.72 |
| Histidine | 1.5 | 1.5 | 0.33 |
| Isoleucine | 4 | 3 | 0.67 |
| Leucine | 4.5 | 4.5 | 1.00 |
| Lysine | 3 | 4.5 | 1.00 |
| Methionine & cysteine | 1.5 | 1.5 | 0.33 |
| Phenylalanine + tyrosine | 2.5 | 4 | 0.89 |
| Threonine | 3 | 3 | 0.67 |
| Tryptophan | 1 | 1 | 0.22 |
| Valine | 4 | 3.5 | 0.78 |
OK, that’s a bunch of numbers, but I suspect that what you’d really want to know is how do my suggested ratios compare to those of the top-performing pollen subs in my trial? See Figure 7.
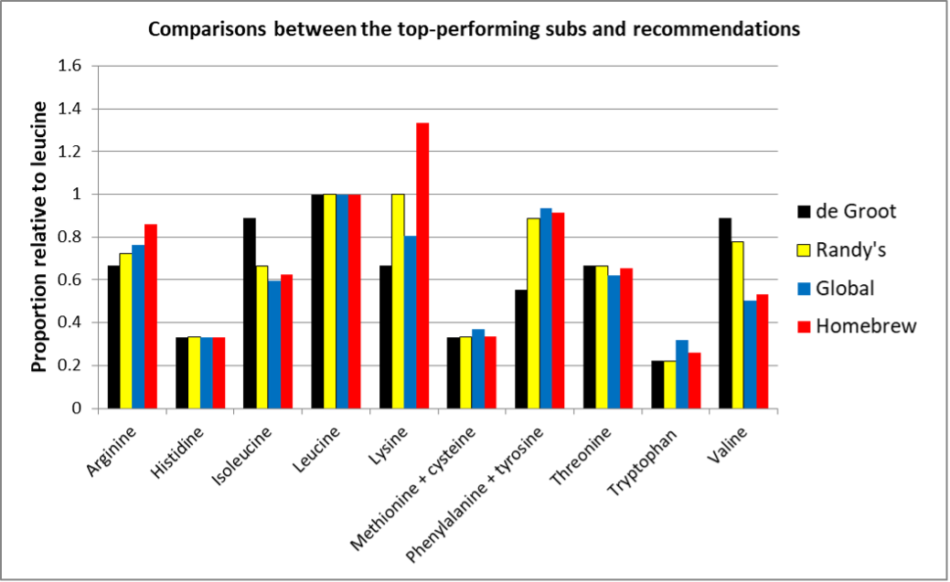
Fig. 7 A comparison of the EAA ratios, normalized to leucine, from de Groot’s suggestion, my suggestion, and the two top-performing tested pollen subs. Note how closely my suggested ratios (yellow) match those of the top performers.
CAGE AND SEMI-FIELD STUDIES
In-lab cage studies are limited by the difficulty of maintaining the necessary jelly-producing nurse bees and rearing the produced brood to maturity for several generations on artificial diets. I’m in complete agreement with Hendricksma [[33]] that:
We suggest that colony supplementation should target nurse bee nutrition. … Our study also highlights the importance of testing honey bee nutrition with field and laboratory studies. Testing diet treatments on caged bees as compared with whole colonies may yield different insights.
Practical application: The gold standard semi-field bioassay for testing the nutritional efficiency of honey bee diets is at the colony level by using nucs with free-flight access in screened cages (4x4x8 feet), developed at the Beltsville Lab [[34]]. I strongly encourage more research on artificial diets using this method.
Suggestions for researchers: That said, I’m curious as to whether a modification of Haydak’s method of extending nurse longevity by removing sealed brood [[35]], or the swarm box method for producing multiple rounds of queen cells from a cohort of nurse bees could be used [[36]].
BASIC FORMULA FOR HONEY BEE ARTIFICIAL DIETS
Based upon a review of the literature, plus the findings from our field trial, one can arrive at suggested proportions for an optimal artificial diet for stimulating brood production.
Crude protein: The best-performing subs were in the 15-20% range (wet weight of the diet), although other research suggests that 25-30% may be optimal [[37], [38]]. EAAs should make up about 50% of the crude protein content. One can use my calculator [[39]] to balance the EAAs by adding concentrates.
Sugar content: around 40-50%.
Lipid content: in the 1-5% range, with canola oil seeming to work well. Ghosh found that palmitic, stearic and oleic acids were predominant in all of the stages.
Vitamins, minerals, and sterols: Dietary requirements are not really clear, and these may be adequately provided by the raw feedstuffs (such as brewer’s yeast). But note that at least some brewer’s yeasts may be distasteful to bees [[40]]. Ghosh found that adult bees contain higher concentrations of the trace elements iron, zinc, calcium, magnesium, and copper than those found in red meat, suggesting that any pollen sub should contain ample levels of these trace elements. It may be wise to make sure that zinc is in the 75 ppm range.
Particle size: most bee-collected pollens are less than 50 micrometers in diameter [[41]], suggesting that all feedstuffs should likely be ground to not larger than that particle size. (I’ve observed colonies kicking out, rather than consuming, larger particles.)
Natural pollen: Again, any benefit is not clear. De Groot found that the enzymes in honey or beebread appeared not to be essential in a diet [[42]]. For phagostimulation, pollen needs to make up not more than 5% of the patty (Figure 8).

An additional photo.

Fig. 8 We’ve heard claims that a high proportion of natural pollen in a patty results in the bees consuming it much more quickly. So this fall my son Eric ran a test, by placing one 4% pollen and one 15% pollen Global patty side-by-side on 150 hives. He then checked back, taking photos at all stages of consumption, and showed them to me as a blinded grader of consumption rate. Neither of us could see any difference.
THAT’S IT!
This has been a much longer write up on pollen subs than I imagined, but since so many of us professional beekeepers spend a great deal of money on them, I hope that ABJ’s readers feel that this series has been informative. I especially hope that what we’ve learned, as well as my remaining questions, encourages our manufacturers to improve their formulations, and our researchers to take up where de Groot and Elton Herbert, Jr. left off.
DE GROOT’S PAPER
I’ve mentioned that de Groot’s monumental study is difficult to access. Those interested can download a copy at https://scientificbeekeeping.com/de-groot-1953/
REFERENCES AND NOTES
[1] Ghosh, S, et al. (2016) Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. Journal of Asia-Pacific Entomology 19: 487–495.
[2] Mitchell, H, in Albanese, A (1950) Protein and amino acid requirements of mammals. Academic Press.
[3] Church, D, et al. (2020) Essential amino acids and protein synthesis: insights into maximizing the muscle and whole-body response to feeding. Nutrients 12, 3717.
[4] Chan, Q & L Foster (2008) Changes in protein expression during honey bee larval development. Genome Biol. 9(10): R156.
[5] Corby-Harris V, et al. (2019) Fat body lipolysis connects poor nutrition to hypopharyngeal gland degradation in Apis mellifera. J Insect Physiol. 116:1-9.
[6] Haydak, M (1970) Honey Bee Nutrition. Annual Review of Entomology 15:143-156. Haydak found that 45% of abdominal nitrogen may be lost by long-lived workers starved for protein.
[7] https://scientificbeekeeping.com/where-does-fed-pollen-sub-go/
[8] Noordyke, E, et al. (2021) Tracing the fate of pollen substitute patties in western honey bee (Hymenoptera: Apidae) Colonies. Journal of Economic Entomology 114(4): 1421–1430.
[9] Haydak, M. H. (1935). Brood rearing by honey bees confined to a pure carbohydrate diet. Journal of Economic Entomology, 28, 657-660.
[10] Otis, G, et al. (2004) Storage proteins in winter honey bees. Apiacata 38: 352-357.
[11] Scheller K, et al. (1990) Molecular properties, functions and developmentally regulated biosynthesis of arylphorin in Calliphora vicina. In: Hagedorn H, et al. (eds) Molecular Insect Science.
[12] Johansson, T & M Johansson (1977) Beekeeping techniques, Feeding Sugar to Bees. 3. Dry Sugar and Candy. Bee World, 58:2, 49-52.
[13] The “aromatic” amino acids listed later in this artlcle.
[14] Jeffree, E (1956) Winter brood and pollen in honeybee colonies. Ins. Soc 3: 417–422.
[15] Haydak, M (1934) Changes in total nitrogen content during the life of the imago of the worker honeybee. J. Agricultural Research 49(1): 21-28.
[16] Straus, J (1911) Die chemische zusammensetzung der arbeitsbienen und drohnen während verschiedenen entwicklungsstadien. Ztschr. Biol. 56: 347-397.
[17] De Groot, A (1954) On the ovary development in queenless worker bees (Apis mellifica L.) Experientia Vol. X/9: 384-385.
[18] Babendreier, D, et al. (2004) Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants. Apidologie 35: 293–300.
[19] Schmehl, D, et al. (2016). Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. Journal of Apicultural Research 55(2): 113-129. I’ve corresponded with Dr. Schmehl about his inclusion of yeast extract — other researchers have found that it’s not necessary.
[20] Zhang, J, et al. (2009) Determination of tryptophan in bee pollen and royal jelly by high-performance liquid chromatography with fluorescence detection. Biomed. Chromatogr. 23: 994–998.
[21] Webster, T, et al. (1987). Conservation of nutrients in larval tissue by cannibalizing honey bees. Physiological entomology 12(2): 225-231.
[22] Loper, G & R Berdel (1980) The effect of nine pollen diets on brood rearing of honey bees. Apidologie 11: 351-359.
[23] de Groot, A. (1953) Protein and amino acid requirements of the honey bee. Physiol. Comp. Oecol. 3, 197–285.
[24] Standifer, L, et al. (1960) Relative availability of various proteins to the honey bee. Annals of the Entomological Society of America 53(5): 618–625.
[25] Williams, H, et al. (1954) Estimation of growth requirements for amino acids by assay of the carcass. J. Biol. Chem 208: 277-256.
[26] Ericson, L (1961) A criticism of the carcass analysis procedure for the determination of amino acid requirements. Acta physiol. Scand. 52: 90-98.
[27] Sklan D & Y Noy (2005) Direct determination of optimal amino acid intake for maintenance and growth in broilers. Poultry Science 84(3): 412-418.
[28] Ghosh, S, et al. (2016) Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable protein source. Journal of Asia-Pacific Entomology 19(2): 487-495.
[29] de Groot, op. cit.
[30] Standifer, L, et al. (1960) Relative availability of various proteins to the honey bee. Annals of the Entomological Society of America 53(5): 618–625.
[31] McNally, J, et al. (1965). Partition of excreted nitrogen from honey bees fed various proteins. The Journal of nutrition 85(1): 113-116.
[32] The basis for his recommended EAA ratios.
[33] Hendriksma, H, et al. (2019) Effects of essential amino acid supplementation to promote honey bee gland and muscle development in cages and colonies. Journal of Insect Physiology 117, 103906.
[34] Herbert E & H. Shimanuki (1978) Effect of the size of outdoor flight cages on brood rearing and food consumption by honeybees. Journal of Apicultural Res 17(3): 114-117.
[35] Haydak, MH (1963) Age of nurse bees and brood rearing. Journal of Apicultural Research 2(2):101-103. Haydak was able to get a cohort of nurses to continually rear larvae for up to 138 days. One could test the nutritional performance of artificial diets by this method.
[36] Laidlaw, H & R Page (1997) Queen Rearing and Bee Breeding. Wicwas Press. It might be possible to measure how many healthy queens could be raised by a cohort of nurses fed an artificial diet.
[37] Herbert, E, et al. (1977). Optimum protein levels required by honey bees (Hymenoptera, Apidae) to initiate and maintain brood rearing. Apidologie 8(2): 141-146.
[38] Zheng, B, et al. (2014). The effects of dietary protein levels on the population growth, performance, and physiology of honey bee workers during early spring. Journal of Insect Science, 14(1).
[39] https://scientificbeekeeping.com/7333-2/
[40] https://scientificbeekeeping.com/a-comparative-test-of-the-pollen-sub/
[41] Hao, K, et al. (2020) Pollen grain size associated with pollinator feeding strategy. Proc. R. Soc. B 287: 20201191.
[42] De Groot, A (1950) The influence of temperature and kind of food on the increase in the nitrogen content of the young worker honeybee (Apis mellifica L.). 45th communication of the “Werkgemeenschap voor Endocrinologie”